1. Benthos Ecology Department, Institute of Marine Biological Research (IMBR) Russian Academy of Sciences (RAS), Nakhimov av., 2, Sevastopol, 299011, Russian Federation
2. Department of Radiation and Chemical Biology, Institute of Marine Biological Research (IMBR) Russian Academy of Sciences (RAS), Nakhimov av., 2, Sevastopol, 299011, Russian Federation

Author

Correspondence author
International Journal of Marine Science, 2016, Vol. 6, No. 11 doi: 10.5376/ijms.2016.06.0011
Received: 16 Feb., 2016 Accepted: 26 Mar., 2016 Published: 26 Mar., 2016
Bondarev I.P., and Malakhova L.V., 2016, The total concentration of carotenoids in Rapana venosa gonad, International Journal of Marine Science, 6(11): 1-7 (doi: 10.5376/ijms.2016.06.0011)
For the first time we investigated the total carotenoid content (TCC) in the gonads predator Rapana venosa depending on the age of the individuals and TCC victim's body. The data obtained show a direct link between the increase amounts of carotenoids in the gonads R. venosa with age. Strengthening the color intensity of the gonads is also associated with an increase in carotenoid content. The content of carotenoids in the Rapana gonade also dependent from TCC of its food items tissue.
Introduction
Carotenoids - an extremely diverse group of pigments with a significant range of biological functions and is widespread in nature. It is considered that this is due to their antioxidant properties that allow neutralizing active forms of oxygen and free radicals. The biosynthesis of carotenoids is carried out by different taxonomic groups of producers (cyanobacteria, algae, plants). Marine animals, especially bivalves (oysters, clams, scallops, etc.) contain various carotenoids, which show structural diversity. Bivalves accumulate carotenoids from their dietary algae and modify them through metabolic reactions (
Maoka, 2009). A large gastropod
Rapana venosa (
Valenciennes, 1846) is a terminal predator in the Black Sea benthic trophic system and bivalves are its’ main nutritial objects and the only issue of carotenoids in
R. venosa tissue. A scheme of metabolic transformation of carotenoids in system “filtrated mollusk → predatorof “ of
Mytilus galloprovincialis Lamarck, 1819 →
R. venosa has been discussed (
Shulman et al, 2014).
R. venosa is dioecious mollusk with well-defined sexual characteristics. Male has distinctly pronounced penis is usually longer than 20mm. Gonads of males and females occupy the upper part of the visceral bag differs in color. Normally female gonads are yellow but abnormally they has tendency to orange and red color which in male character (
Mann et al., 2006,
Micu et al
., 2009,
Bondarev, 2015). The gonads of males are from orange to dark brown in color (
Bondarev, 2015).
Measurements of the average values of the total carotenoids content (TCC) in different tissues of
R. venosa have shown that the highest concentration (61-65%) is inherent in the gonads of individuals of both sexes. In this carotenoid in the gonads of male content may be 10 times higher than in females (
Shulman et al, 2014). The connection showed of the intensity of pigmentation of the gonads with the age individuals (
Bondarev, 2015).
In this paper we test the hypothesis that the
R. venosa different color gonads have different contents of the main pigments - carotenoids. Of particular interest are the individuals with abnormal color for the species. Abnormal color of the gonads in females in the Crimea we studied populations are not associated with imposex status, and their number is less than 3%, which is consistent with previous studies (
Bondarev, 2015). This amount is significantly less than the areas where the abnormal coloration associated with masculinization, as in the western part of the Black Sea, Romania, Agigea littoral (imposex females up to 30%) (
Micu et al
., 2009) and in Chesapeake Bay (USA) where the preponderance of imposex females is over normal females by at least a 2:1 ratio (
Mann et al., 2006). In these regions
R. venosa imposex females had penis (1.14mm – 25 mm) and yellow to yellow-orange gonads.
The main purpose of this paper is demonstrating dependencies TCC in
R. venosa specimens’ gonad of different color and age. For this purpose we held a series of measurements TCC gonads
R. venosa individuals of various ages and colors of three different populations living in the vicinity of Sevastopol (
Figure 1). For comparison, we studied samples from several places that differ in their orography, bottom sediments and the most important in nutritial resources.
|
.png)
Figure 1 Sampling areas: 1 - Round bay, 2 - Streletskaya bay, 3 - Blue bay
|
Also TCC measurements were made for victim bivalve mollusc Chamelea gallina (Linnaeus, 1758) made it possible to compare the amount of carotenoids in the predator R. venosa according to the food of the object.
1 Results
The resulting 43 measurements TCC in the gonads R. venosa showed a fairly wide range of values (0.58-57.04 mg/100g).
Coloration gonads depend on the amount of brown pigment granules in the sheath tubes containing germ cells. In males, the pigment granules are significantly higher than that of females and determines that the difference in color (
Chuhchin, 1970). Published data TCC in the gonads of male and female indicates their significant difference (6.28-8.2 mg / 100g against 8.54-81.4 mg / 100g, respectively) (
Shulman et al, 2014).
Our studies confirm the TCC sex differences in
R. venosa gonads
. The females with normal gonads from light yellow (
Figure 2A) to bright yellow color have TCC = 0.58-13.23 mg / 100g. In females with abnormal gonads from dark yellow to yellow-orange color (
Figure 2B) TCC is much higher (21.09-45.0 mg / 100g) and the respective characteristic of males. Color gonads of «anomalous» females are close to the color of males, which is quite natural, because carotenoids are the main pigments gonads
R. venosa. The shell length (SL) of anomalous females (59.0-68.5mm) more than the average for the sex (48.3mm) values, and even larger than the average size of males (58.8mm) of our population. A similar situation specified for Chesapeake Bay, where the size-weight characteristics imposex females are closer to the parameters of males than normal females (
Mann et al., 2006).
.png)
Figure 2 R. venosa females’ soft body with normal (A) and abnormal (B) color of gonad (gon) and TCC,
mg/100g = 0.83(A), 45.0(B) |
All females with abnormal coloration of gonads in our recent samples were aged 6-8 years which corresponds our previous study with 5 years age lower limit (
Bondarev, 2015) and the observation made for Chesapeake Bay imposex females were not among young aged individuals (
Mann et al., 2006).
Graph of TCC of age females with normal gonads shows a high value credibility the linear approximation (R² = 0.991) (
Figure 3).
.png)
Figure 3 Graph of TCC of age R. venosa females with gonads normal color
|
Adding to the array of data indicators TCC abnormal individuals significantly distorts the graphic pattern (
Figure 4), and drastically reduces the coefficient of determination (R² = 0.394). This suggests the need for a separate examination of the analytical results for the “normal” and “abnormal” female. Remarkable the highest rate TCC (45.0 and 27.13 mg / 100 g) in our samples are not observed in the oldest female and abnormal specimens at the age of 6 years. At the 8 year older individuals abnormal the TCC is lower (21.09 mg / 100 g).
.png)
Figure 4 Graph of TCC of age females with “normal” and “abnormal” gonads
|
Dependency TCC on the size (SL) of individuals is absent even for a sample consisting of only "normal" females (R² = 0.000, the graph is omitted). Analogous conclusions have previously made for the thickness of the shell, which in today's "mixed" population of the Crimea is also correlated with the age of the individuals, not the size (
Bondarev, 2013). For populations in which the size and age are directly dependent the same direct correlation can be expected for a pair of size - TCC. Color of female gonads and TCC are not related to the color of the shell. Females with abnormal gonads may have a close to an extreme (melanestic) color, as well as typical one for the species. Moreover, the maximum value of abnormal TCC were observed in females with the typical color of the shell (
Figure 5).
.png)
Figure 5 Shells of different color females with TCC anomaly: A – melanestic specimen, SL – 59mm, TCC = 27.1 mg / 100g; B – normally patterned specimen, SL - 60mm, TCC = 45.0 mg / 100g
|
Females with the abnormal color of the gonads were not found in the Round bay that is probably a consequence of local dietary habits Rapana. It is noteworthy that the oldest (12 years) female in our sample was found in the Round bay and had a normal yellow color of the gonad. The main object of Rapana food in the area is
Ch.gallina with low TSS level in the tissue is 2.19-4.05 mg/100g. This rate is significantly lower than that of
M. galloprovincialis which is the principal object of nutrition for Rapana in the Streletskaya bay and partially for the population in the Blue bay. TCC
M. galloprovincialis soft body is 8-16 mg / 100g (
Karnaukhov, 1988) and 17-20 mg / 100g (
Shulman et al, 2014).
Previous studies have shown that the intensity of the color of the males’ gonads
R. venosa increases as age increases. It was shown that there are two principal lines of gonad color: "red" - carotenoid and "brown" - melanin. It was assumed that the type of color depends on the feeding spectrum in which the quantitative content of carotenoids associated with the victims’ taxonomic structure (
Bondarev, 2015). Our present data confirm that the amount of carotenoid in the
R. venosa gonads associated with tissue food objects’ TCC.
The lowest indicators of gonadal TCC characteristic for
R. venosa population of Round bay - 2.18-14.29 mg / 100g at individuals aged 3-6 years. Dependency TCC increase with age is nearly linear (
Figure 6) with high coefficient of determination (R² = 0.907).
.png)
Figure 6 Graph of TCC of the age in the gonad R. venosa male individuals of Round bay
|
The main R. venosa object of feeding in the Round the bay is Ch. gallina with relatively low level of the TCC (2.19-4.05 mg / 100g) in the tissue. Gonad color males R. venosa from the area varies from beige in 3-year-old individuals (with minimum values of TCC = 2.18-6.04 mg / 100 g) until brown at the 6-year-old individuals (TCC = 14.29 mg / 100 g).
Data set
R. venosa the same age range (3-6 years) of the Blue bay shows the widest range of color of the gonads and TCC index (9.07-57.04 mg / 100 g). Information on the gonad color
R. venosa of the bays Blue and Round are consistent with the descriptions and illustrations in our previous study (
Bondarev, 2015). Variety of colors gonads
R. venosa from Blue bay is determined by a wide range of feeding which includes bivalves Veneridae and Mytilidae as well. Since the color intensity of the
R. venosa gonad determined by the presence of brown granules in the gonad tube casing (
Chuhchin, 1970) we would expect the highest values of TCC in individuals with the most dark colored (dark brown) gonads. However, comparison of the TCC values and the color of the gonads show that the highest rates of TCC (57.04 mg / 100 g) in our sample correspond to a brighter red-brown (
Figure 7D). At coeval and even older individuals with dark brown gonads indicators TCC are lower (
Figure 7). This confirms the validity of the conditional division of colors on the male gonads of "red" carotenoid and "brown" melanin (?) lines (Bondarev, 2015). The presence in the color of the gonads shades of red or orange indicate high values of carotenoids. The relatively wide range of TCC values for individuals of the same age (
Figure 7) reflects the diversity of colors shown for Blue bay
R. venosa (
Bondarev, 2015,
Figure 8).
In general the graph of TCC of age
R. venosa Blue bay shows a relatively high degree of correspondence (R² = 0.744) despite the rather wide scatter of data for specific age groups (
Figure 8).
.png)
Figure 7 Soft bodies of different ages R. venosa male individuals with gonads of different colors and TCC, mg / 100 g: A – 5 year, 32.37 mg/100g; B – 7 year, 39.36 mg/100g; C – 6 year, 49.17 mg/100g; D – 6 year, 57.04 mg/100g
|
.png)
Figure 8 Graph of TCC in the gonad of the age of male individuals’ R. venosa Blue bay
|
Somewhat unexpected results have been obtained for
R. venosa collected in the Streletskaya bay on rocky ground with
M. galloprovincialis. It was assumed that the feeding object with a relatively high content of carotenoids must complies predator
R. venosa with also high TCC and red and orange tones in the color of the gonads males. However, all the males in this sample had the gonads colored from brown to dark brown. And their indexes TCC (25.94-38.47 mg / 100 g) were lower than for the same age individuals from Blue bay with mixed feeding, although it is higher than that of Round bay
R. venosa TCC range with
Ch. gallina nutrition. This phenomenon can offer the following explanation. Because within the Streletskaya bay rocky biotope were found individuals not younger then 6 (up to 9) years and the size of 70-97mm, it can be assumed that we are dealing with individuals which moved from the sand area where they were fed
Ch. gallina. When reaching large size the predator needs a larger prey (
M. galloprovincialis), which increases the efficiency energy. Thus, the accumulation of carotenoids in this group began more actively just in the last years of life and did not reach the expected high level. TCC tested
R. venosa species of the Streletskaya bay expectedly increased with age (R² = 0.795) (
Figure 9).
.png)
Figure 9 Graph of the TCC in the gonads from the age of male individuals’ R. venosa Streletskaya bay
|
2 Discussion
The ecological biochemistry of marine organisms change in carotenoid concentrations in aquatic organisms is considered as one of the molecular mechanisms of adaptation to the effects of negative environmental factors (
Karnaukhov, 1988). For example, the greatest amount of carotenoids (8-16.0 mg / 100 g) recorded in young animals
M. galloprovincialis a shell length of 1.0 cm. In process of growth mollusc content of these biologically active compounds decreased to 1.5-2 times. During aging mussel carotenoid content rises again to 9.0 mg / 100 g. Carotenoids accumulation with age is a process of adaptation growing old tissues of organisms to progressive interstitial hypoxia (
Karnaukhov, 1988).
Our data show a clear tendency of accumulation of carotenoids in organism with increasing age of the individuals in each of the populations studied. But for R. venosa this process is ongoing progressive.
At the same time, when considering the total set of male
R. venosa from all 3 investigated areas notes that the oldest individuals are not the highest TCC gonads (
Figure 10). The highest rate of TCC in our collections is not marked in the oldest individuals but the individuals of the age of 6 years old (57.04 mg / 100 g). Older 8-9 year olds TCC lower (28.91-38.47 mg / 100 g).
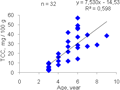
Figure 10 Graph of TCC in the males’ gonad of the age of individuals’ R. venosa from sampling areas 1, 2, 3
|
The same holds with respect to size (SL) R. venosa. Males 6-9 years of age, SL - 82-97mm of the Streletskaya bay have TCC (25.94-38.47 mg / 100 g) is 1.5-2 times lower than 6 year olds from Blue bay (SL - 48.2-52.5mm, TSS = 45.75-57.04 mg / 100 g). The oldest specimen (female 12 years) in the 2015 samples was found in the Round bay, where R. venosa and the object of her nutrition (Ch. gallina) are characterized by the lowest values of the TCC. This female had normal yellow gonads (TCC not measured) and the size (SL) of 75 mm, which is larger than the "anomalous" females from other populations. In the Round bay "anomalous" females were not found. Thus the size of Rapana (SL) of the Round bay (45.5-89mm) is larger than the size of the individuals of the Blue bay samples (31.3-74.0 mm), where the rates of TCC R. venosa maximum. Perhaps the producents of the most open sea Blue bay differ in species composition, in which more carotene generating organisms, with the base of the food less abundant than in the other two studied bays. Sexual structure of populations shows that the most balanced sex ratio (1: 1) was observed for the Round bay, while the local population of the Blue Bay the sex ratio is approximately 1: 2 in favor of males. The number of individuals older than 5 years in the Round bay in 2015 was 25%, and in the Blue bay - 13%. These data suggest that the Round bay population of R. venosa environmentally more favorable, despite the relatively low values of the TCC in the tissues of victims and predators gonads.
The highest TCC rates of male
R. venosa (up to 81.4 mg / 100 g) provided for the area of Karadag (Eastern Crimea) (
Shulman et al, 2014). These data were obtained in 2008, and in 2009 in the area conducted
R. venosa population studies (
Bondarev, 2010). In the area of Karadag in 2009
R. venosa male gonads were various shades of orange that is a testament to the high level of TCC. In the age structure of the population of
R. venosa individuals older than 5 years old was 3.2% of the total, and the maximum age of the individuals was 10 years old. Sex ratio was 1: 1.86 in favor of males (
Bondarev, 2010).
Thus, the high level of TSS is not always the main indicator of the capacity of the physical growth and longevity of the organism. Since consuments different levels: mollusks -filter feeders and predators get carotenoids on the food chain of the objects of plant origin, the content of these pigments depend on the levels in the producents. The accumulation process of carotenoids in R. venosa runs progressively throughout the lives, but in the framework of the opportunities offered by a particular local trophic chain. The mechanism of formation anomalous TCC values in the R. venosa gonads requires extra material for research.
3 Material and Methods
Collection of samples for this study was carried out in the vicinity of the city of Sevastopol in summer-autumn (27 June – 06 October) 2015. Sampling conducted at depths of 2 - 6 meter in ecologically different habitats: 1- open shallow bay in the city, sand bottom (Round Bay), 2 - open moderate-deep bay in the city, rocky bottom (Streletskaya Bay), 3- open bay on the border of the city limits, sand bottom (Blue bay) (
Figure 1). To determine size, sex and age structure of populations 81, 13 and 162 specimen were collected, respectively, specified areas.
From each population were selected individuals with gonads of different colors to measure total carotenoid content (TCC). The number of R. venosa individuals examined for TCC by areas was as follows: Round bay – 20, Streletskaya Bay – 5, Blue Bay - 18 specimens. 3 tested Ch. gallina specimens were taken from the Round bay.
Extraction of total carotenoids and TCC determination made for 46
R. venosa and 3
Ch. gallina specimens. The extraction of total carotenoid was employed according to the reported method (
Yanar et al., 2004) with some modifications. To avoid degradation of carotenoids, the extractions were performed in an aphotic environment. Sample 0.1 g wet weight homogenised gonads was mixed with equal amounts of anhydrous sodium sulfate and the carotenoids were then extracted in 5 ml acetone during 3 h in the dark in a refrigerator at 4ºC. Following extraction, the mixtures were centrifuged at 5000 rpm for 5 min. The resulting supernatant was separated. Absorbance of the extracts was measured at 450 nm in a Helios Alpha and Beta UV-Visible Spectrophotometer (Thermo Scientific, USA). TCC was calculated using an extinction coefficient E (1%, 1 cm) of 1.900 (
Foss et al., 1987;
Yanar et al., 2004). TCC analysis was performed in duplicate; the results were expressed in mg/100 g tissue on a wet weight basis.
Total
R. venosa shell length (SL) was measured using a caliper (0.1mm). Each whelk was classified as male (m) or female (f) by penis presence/absence respectively and gonad color. Individual age
R. venosa specimens determined based on annual spawning marks on the shell in accordance with the method proposed Chuhchin V.D. (1961a,b) as represented (
Bondarev, 2010). To more accurately determine the age the specimen shell must be cleaned of various types of surface growths as noted in (
Bondarev, 2015).
Photographing tissue samples carried out directly after removing the soft body from the shell. The shells pictured after cleaning operations using digital camera.
Bondarev I. P., 2010, Shell morphogenesis and intraspecific differentiation of Rapana venosa (Valenciennes, 1846), Ruthenica, 20 (2): 69-90 (in Russian)
Bondarev I.P., 2013, Ecomorphological Analyses of Marine Mollusks’ Shell Thickness of Rapana venosa (VALENCIENNES, 1846) (Gastropoda: Muricidae), International Journal of Marine Science, 3: 368-388
Bondarev I.P., 2015, Sexual differentiation and variations sexual characteristics Rapana venosa (Valenciennes, 1846), International Journal of Marine Science, Vol.5, No.19: 1-10
Chuhchin V.D., 1970, Functional morphology of rapana, Kiev, Naukova Dumka, 139p (in Russian)
Foss, P., Storebakken, T., Austreng, E., Liaaenjensen, S., 1987, Carotenoids in diets for salmonids: V. Pigmentation of rainbow trout and sea trout with astaxanthin and astaxanthin dipalmitate in comparison with canthaxanthin, Aquaculture, 65: 293–305.
Karnaukhov V.N., 1988, The biological functions of carotenoids, Moscow: Nauka, 240 p (in Russian)
Lanfrankoni A., Hutton M., Brugnoli E., Muniz P., 2009, New record of the alien mollusc Rapana venosa (Valenciennes 1846)in the Uruguayan coastal zone of Rio de la Plata, Pan-American Journal of Aquatic Sciences, 4(2): 216-221
Mann R., Harding J.M. and Westcott E., 2006, Occurrence of imposex and seasonal patterns of gametogenesis in the invading veined rapa whelk Rapana venosa from Chesapeake Bay, USA, Marine Ecology Progress Series310: 129–138
Maoka T., 2009, Recent progress in structural studies of carotenoids in animals and plants, Archives of Biochemistry and Biophysics, 483: 191–195
Micu S., Comanescu G., Kelemen B., 2009, The imposex status in Rapana venosa population from Agigea littoral. Analele ȘtiinÈ›ifice ale Universității „Al. I. Cuza” IaÈ™i, s. Biologie animală, T. LV: 133-137
Shulman G. E., Soldatov A.A. (Editors), 2014, Black sea molluscs: elements of comparative and environmental biochemistry. Sevastopol: EKOSI-Gidrofisika, 323p, 138 ill., 63 tables (In Russian)
Yanar, Y., Celik,M., Yanar, M., 2004., Seasonal changes in total carotenoid contents of wild marine shrimps (Penaeus semisulcatus and Metapenaeus monoceros) inhabiting the eastern Mediterranean, Food Chemistry, 88: 267–269.
 Author
Author  Correspondence author
Correspondence author
.png)
.png)
.png)
.png)
.png)
.png)
.png)

.png)


